
Boron Neutron Capture Therapy (Part II) – Industrial Aspects

One of the methods proposed was boron neutron capture therapy (BNCT) which used boronophenylalanine as a boron-10 delivery agent and neutrons from the core of a nuclear reactor. The first clinical investigation and treatments were conducted in the US, in the 1950s, with encouraging outcomes, but the technology was far from ready for rapid clinical advancement of BNCT.
For long decades, BNCT was restricted to small and patient-unfriendly research facilities at nuclear reactors. But in the last ten years, the radiation-therapy industry has ushered in a revival of BNCT with the development of compact accelerator-based neutrons sources and the validation of boron-10 carrier compounds. Clinical research is now rapidly growing, and BNCT is finally getting ready for clinical use worldwide.
With nine expert panellists, Cosylab’s March 2022 educational webinar on boron neutron capture therapy (BNCT) attracted almost 500 participants. The event illuminated the history, technology, clinical aspects, and industrial perspective of BNCT and gave attention to its future.
In this blog, we focus on the experts’ views on the industrial development, boron-10 delivery drug research, and challenges of BNCT, including the essential future collaboration between clinicians and industry to fully enable BNCT in the hospital. To learn more about the clinical aspects of boron neutron capture therapy and the essentials of BNCT, see the first blog.
So, how can the industry help oncology adopt BNCT as an everyday therapy modality at a hospital near you?
The Industrial Aspect of BNCT
In the second part of the webinar called “Today’s Use and Future Aspects of Boron Neutron Capture Therapy“, Dr Niek Schreuder, Chief Scientific Officer at LEO Cancer Care, describes the commercial landscape of BNCT.
Currently, there are about twelve different industrial groups involved in manufacturing devices — or providing various forms of technology — for BNCT. Three of them took part in the webinar, namely Neutron Therapeutics, TAE Life Sciences and Sumitomo Heavy Industries. Their presentations help clarify the technical and industrial aspects of BNCT’s transition to the oncology ward.
Sumitomo Heavy Industries and BNCT
Toshinori Mitsumoto, the principal engineer from Sumitomo, explains that his company provides modern systems for diagnosing and treating cancer with medical accelerators. There are three Sumitomo BNCT systems in operation, all in Japan, one in a non-clinical research mode and two used to treat patients.
In March 2020, Sumitomo obtained Japanese medical-device approval to manufacture and sell accelerator-based BNCT systems and dose-calculation applications. In the same year, Japan accepted BNCT treatment for unresectable locally advanced or locally recurrent head and neck cancer as a part of healthcare insurance coverage. This helped increase the annual number of BNCT fractions in Japan, and, to date, more than 160 patients have been treated with Sumimoto BNCT technology.

Mr Mitsumoto describes how his company’s BNCT system, called NeuCure, can spatially fit into a clinical four-room layout in a hospital wing. The Device room contains the beam-line, beryllium target, neutron moderator, and the device’s heart — the 30 MeV cyclotron accelerator. The latter accelerates a beam of protons with a one mA current and is well-tested for hospital use in more than 200 PET drug-synthesis and proton therapy systems
The Preparation room of a NeuCure ward contains the starting point of the floor railing and the “docking” position for the patient-transport system, which consists of the mobile patient bed and chair (seated and supine treatment table).
Sumimoto’ Solution for Patient Transport and Positioning
Technicians take care of the time-consuming patient positioning and restraining in the Preparation room, which is always radiation-free. Then the patient positioner automatically moves the patient to the adjacent Treatment room without any accompanying staff.
In the Treatment room, the patient positioner stops in the correct spot in front of the beam-port of BNTC collimator assembly, with the patient set up in the optimal body position demanded by the treatment area. The clinical team can position Sumimoto’s treatment table to optimise the efficacy of the neutron flux by minimising the air gap, specifically in the seated configuration for treating head and neck cancer and in the supine position for irradiating the brain.
The company’s patented neutron-dose control uses proton-current monitors to deliver an accurate dose from the beryllium target by a beam with more than a 1.0 x 10^9 / (cm2 s) epi-thermal neutron flux.
Mr Mitsumoto also points out that Sumimoto’s new extended collimators for the NeuCure system have been approved in 2022, allowing for more natural patient posture while retaining a high neutron flux. His company is part of the Japanese BNCT effort to get recurrent malignant glioma and malignant meningioma accepted as soon as possible for national health system reimbursement.
Neutron Therapeutics and nuBeam
Dr Elizabeth Reczek is the CEO of Neutron Therapeutics, Inc. from the US. She introduces her company’s nuBeam technology as a BNCT system with the highest flux and the only one compliant with IAEA standards for the clinical use of neutrons. Neutron Therapeutics benefited from starting with a mature accelerator technology with a track record of safe and reliable operation in the industrial setting.
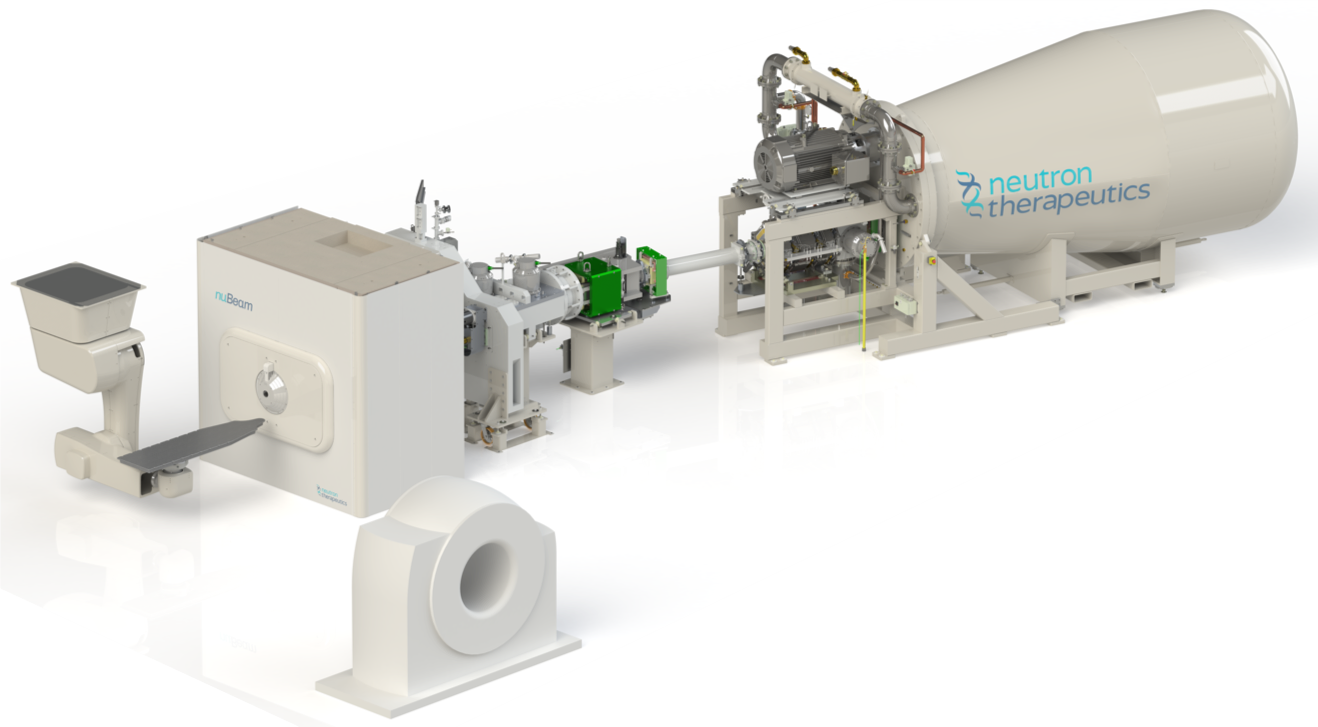
The company had several clear goals for the nuBeam system: minimised exposure rates, highest radiation safety for the clinical staff, and the least possible downtime and risk to staff while servicing and maintaining the system. Furthermore, the entire BNCT beam system had to fit within several rooms of a traditional radiation therapy clinic — with 230 m2 needed for the device’s equipment and an additional 110 m2 of ancillary space.
The aim for the optimised neutron beam was also to allow for significantly shorter treatment times for patients than with previous BNCT devices, with at most two treatment fractions needed for complete therapy.
nuBeam is now a clinical solution for boron neutron capture therapy with all components necessary for imaging, treatment planning, patient positioning and treatment. The system’s source uses a 2.6 MeV electrostatic proton accelerator operating at 30 mA and a rotating, solid lithium target for generating a homogenous beam of epithermal neutrons.
The beam-shaping assembly and a neutron generating target also provide shielding to protect the patient and staff. A positioning robot and in-room CT scanner provide image-guided treatment in real-time.
BNCT in Helsinki
Finland has been a European hotspot of BNCT trials since 1992 but had to close down its FiR 1 nuclear reactor in 2012 due to costs. The excitement is returning to the Finnish oncology community with the installation of Europe’s first accelerator-based BNCT system – the nuBeam solution — at the Helsinki University Hospital. It is currently operating daily for verification and validation testing, with the first clinical trials expected to begin in 2023.
Safety during target exchange was another critical consideration during the design of nuBeam. The system makes the lithium target exchange secure, cost-effective and routine. It utilises its ceiling-mounted patient-positioning robot to remove the spent targets, place them in the lead line storage bins for disposal, and then fit new targets.
This elegant solution eliminates technician exposure and interferes with the treatment schedule the least. Spent targets need to be handled with caution and stored for a while before they can be reconditioned and reused.
Dr Reczek concludes that the extensive testing at the Helsinki University site has demonstrated nuBeam’s capability to sustain the target proton current for extended periods, up to 20 to 40 hours per week, for sixteen consecutive months.
Recent BNCT drug development and use overview
The Chief Medical Officer at Neutron Therapeutics, Dr Seppo Pakala concentrated on a general overview of boron-10 delivery carriers by emphasising their fundamental requirements.
Treatment with boron-10 carriers yields a therapeutic effect only when we achieve a high enough boron-10 concentration in the cancer cells of the specifically targeted tumours.
Of course, the targeted cells must also retain high enough levels of boron-10 concentration to allow efficient neutron irradiation. On the other hand, the boron-10 load to normal cells should be very low, and its clearance from blood rapid to avoid harmful effects in normal tissue. Clinical trials show that a therapeutic effect can be achieved if the carrier content in the tumour is at least three times higher than in normal tissue.
The challenge for developers of the novel delivery substances is now to satisfy the clinical-use requirements as soon as possible. These agents will achieve much higher tumour-to-normal tissue ratios, with which we could shorten the iteration time and treat more patients per day.
Clinical requirements for New Delivery Carriers
Today’s most commonly used boron-10 carrier, boronophenylalanine (BPA) fructose, is produced in hospital pharmacies separately for each patient and with quality verification of each batch, which is time-consuming. New boron-10 carriers should be registered drugs with long shelf-life, produced with good manufacturing practice (GMP), with no extra workload for pharmacies, easily administered to the patient and with no need for supplementary treatments.
Additionally, boron-10 dosing could be improved by moving from 18F-labeling of BPA to direct, in vivo detection of boron-10 concentration in tumour cells. The growth of the installed base of accelerator-based BNCT devices and the number of efficient clinical teams worldwide is reaching critical mass for BNCT adoption in conventional cancer therapy. The expansion of indications for BNCT will also make pharma companies more interested in boron-10 carrier development. Two companies actively developing new boron-10 carriers are TAE Life Sciences and Tenboron.
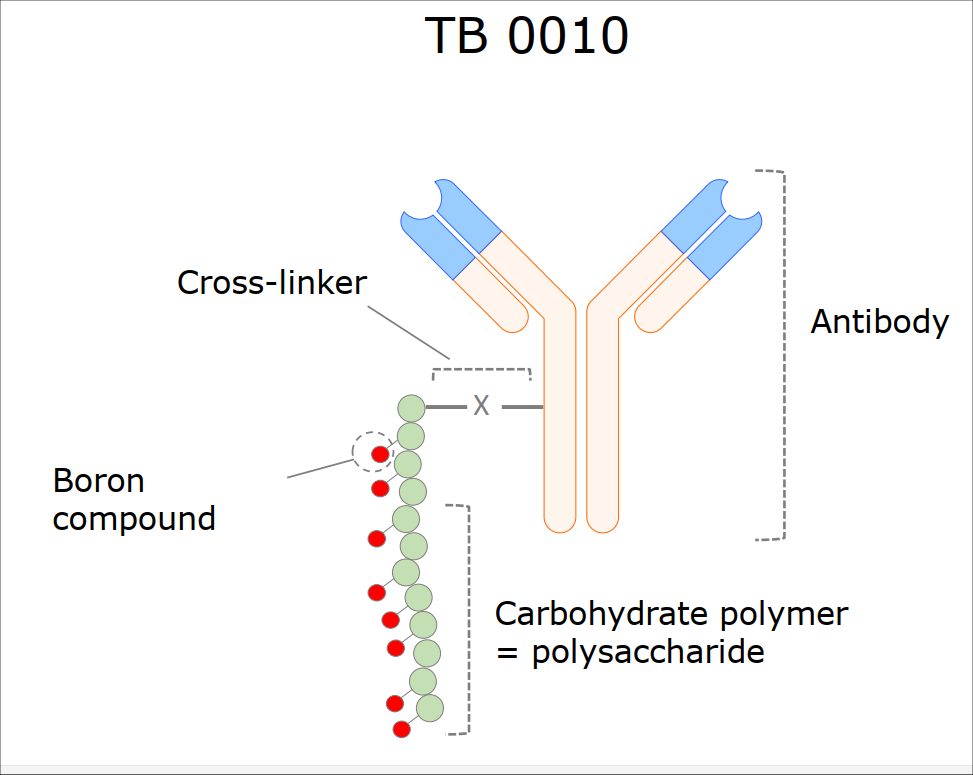
The Finnish Tenboron has three carriers in its development pipeline — a BPA-like non-specific carrier, a tumour-protein targeted carrier for glioblastoma, and the antibody boron conjugate for HER2 positive tumours, the TB0010. The latter is the furthest in the pipeline and has been successfully tested in pre-clinical studies in xenograft mice and rats, achieving in-tumour tissue concentrations of 40 ug/g to 100 ug/g. With a tumour-to-blood ratio above 90 and an in-tumour retention time longer than a day, TB0010 is now ready for Phase 1 studies.
Dr Pakala concludes that current boron-10 carriers have been well able to demonstrate the commercial proof of the BNCT concept. Clinical results with BPA show very promising results in several indications, such as:
- recurrent head and neck tumours;
- primary therapy in non-operable head and neck tumours;
- glioblastoma;
- meningioma and
- melanoma.
Nevertheless, he believes that now is the time for biotech companies to invest in the innovative boron-10 carrier market and start developing even more efficient substances.
TAE Life Sciences and New Carriers
Robert Hill, the COO of TAE Life Sciences (TLS), presented his company as a supplier of a complete BNCT solution — both the neutron-producing accelerator and the biological carriers of boron-10.
Regarding the latter, TLS is focused on two primary areas. One is developing small molecules that are in many ways similar to the current boron-10 delivery drug BPA. The second is working on newer generation carriers called antibody boron conjugates (ABCs). Because these are large, they can transport high numbers of boron-10 atoms to cancerous cells that express as targets.
Unique boron-enriched linkers (BELs) have the potential to deliver hundreds of boron atoms per antibody without affecting the healthy-cell uptake of the boron-10. ABC may not only improve boron-10 concentrations in targets but also expand the cancer indications treatable with BNCT.
Closer to clinical use is, however, TAE Life Science’s small molecule program. These next-generation “BPA-like” agents are moving towards the end stage — getting prepared to be administered to patients in a clinical setting. Because of their small size, amino-acid analogues can easily cross the blood-brain barrier and effectively deliver boron-10 to targets that contribute to brain cancers.
Two of TLS’s candidates, TC 220 and TC 221, have consistently delivered more boron-10 than BPA in all the tested models, i.e. a 2.5 times improvement in the blood ratio and a 3.6 increase concerning brain delivery. As a result, clinicians can escalate doses and achieve shorter treatment times while treating tumours at deeper levels
Tae Life Sciences tested the TC 220 and TC 221 in collaboration with Kyoto University, which performed protocoled BNCT treatment on mice with colon cancer. The experiment showed that the two compounds inhibited tumour growth much better than BPA. In fact, on the 40th day after the one BNCT fraction, there was no evidence of mice tumour left, only scar tissue.
TAE Life Sciences’ Device
Mr Hill mentions with pride that in September 2021, his company had — in partnership with the Neuboron Medical Group — installed its first accelerator-based BNCT device at the most advanced BNCT facility in China, the Xiamen Humanity Hospital.

The facility is designed with three treatment rooms. TLS’s device — the Alphabeam System — includes a compact electrostatic tandem accelerator that speeds protons to energies of 2.5 MeV with a current of 10+ mA. Magnets then steer and focus the beam to the appropriate treatment room, where a proprietary, solid lithium neutron target intercepts the beam and emits epithermal neutrons through an innovative beam-shaper.
Besides its plans to assemble up to twelve BNCT installations globally over the next five years through various partnerships, TAE Life Sciences’ strategic goal is to test its new BNCT boron-10 carriers as broadly as possible, in cooperation with all research groups and BNCT-device companies in the world. The sooner these boron-10 substances reach the market, the better for millions of patients.
Towards a Future of BNCT Clinical-industrial Partnership
Dr Niek Schreuder wrapped up the webinar by pointing out the excitement of the radiation therapy community about the future in which boron neutron capture therapy can be delivered to patients in a hospital setting by a compact irradiating device, far away from the nuclear reactors of yesteryear.
The Particle Therapy Co-Operative Group (PTCOG) was founded in 1985 and is a non-profit worldwide organisation of scientists and professionals interested in the development of particle radiation therapy. Dr Schreuder believes that PTCOG may help bring the oncological efforts of clinical teams and BNCT device manufacturers even closer.
He invited everybody interested in contributing to BNCT technology and know-how to contact the BNCT subcommittee of PTCOG in advance of this year’s meeting in Miami in June 2022.
The closer clinicians, medical physicists and engineers cooperate, the sooner a BNCT device in the clinic nearby will start saving lives by irradiating just the cancer cells.
Note
The webinar “Today’s Use and Future Aspects of Boron Neutron Capture Therapy” is rich in detail and subject matter. This blog summarises only some of the talking points regarding industrial development and the challenges of BNCT.
In the previous blog, Part I of the webinar, we examined the participating experts’ views on the clinical aspects of BNCT. We will also publish more in-depth content on boron neutron capture therapy in the months to come, as well as organise new educational webinars about the subject.
List of the experts participating in the educational webinar on BNCT on the 2nd of March 2022 (in order of appearance)
- Jay Flanz, PhD (President and Chair of PTCOG, Retired assoc. Prof. at the Harvard Medical School, Retired Technical Director at the Francis H. Burr Proton Therapy Center of the Massachusetts General Hospital);
- Paul Busse, MD, PhD (Joseph W. Cotchett Endowed Chair in Radiation Oncology, Massachusetts General Hospital);
- Wolfgang Sauerwein, Prof. Dr Med (BNCT Global GmbH, Heinrich Heine University Dusseldorf, President of the German Society for Boron Neutron Capture Therapy);
- Thomas Rock Mackie, PhD (Emeritus Professor, University of Wisconsin, Board Chairman and Co-Founder of Leo Cancer Care, Board member of Cosylab, Board member in Shine Medical Technologies);
- Hanna Koivunoro, PhD (Chief Medical Physicist at Neutron Therapeutics Inc.);
- Akira Matsumura, M.D. Ph.D. Dr.med (President, Ibaraki Prefectural University of Health Sciences, Emeritus Professor, Faculty of Medicine, University of Tsukuba, Japan);
- Toshinori Mitsumoto, Principal Engineer at Sumitomo Heavy Industries;
- Elizabeth Reczek, PhD (Chief Executive Officer at Neutron Therapeutics, Inc.);
- Rob Hill (Chief Operating Officer of TAE Life Sciences);
- Seppo Pakala (Chief Medical Officer at Neutron Therapeutics Inc.);
- Niek Schreuder, PhD (Chief Scientific Officer at Leo Cancer Care);
