BNCT Part I: Current and Future Clinical Aspects

With BNCT, the targeted cancer cells are destroyed by alpha particles and 7Li ions that emerge from the 10B atoms absorbing neutrons. BNCT is relatively safer than radioisotope therapy or chemotherapy, where toxicity can reach all parts of the body.
Boron neutron capture therapy shows great promise in treating hard-to-treat, recurring or even metastasised cancers. With the advent of compact and on-site accelerator-based devices, what does BNCT need to take the oncology clinic by storm?
In March 2022, Cosylab organised an educational webinar on BNCT. With nine experts from around the globe as panellists, the event attracted over 450 participants. In this blog, we focus on the clinical aspects of BNCT, while in the next one, we shall examine the experts’ views on Industrial development and challenges of BNCT.
An Introduction to Boron Neutron Capture Therapy
In his opening remarks to the webinar, Dr Jay Flanz, the President and Chair of PTCOG (Particle Therapy Co-Operative Group), underlines that radiotherapy can be efficient only if the target tissue and the healthy tissue are well-differentiated and the radiation be well-targeted.
Boron neutron capture therapy is a highly interdisciplinary activity that requires technology, physics, engineering, biology, chemistry, and of course, medicine and combining all of these pieces has been a challenge. But BNCT is turning a new page!
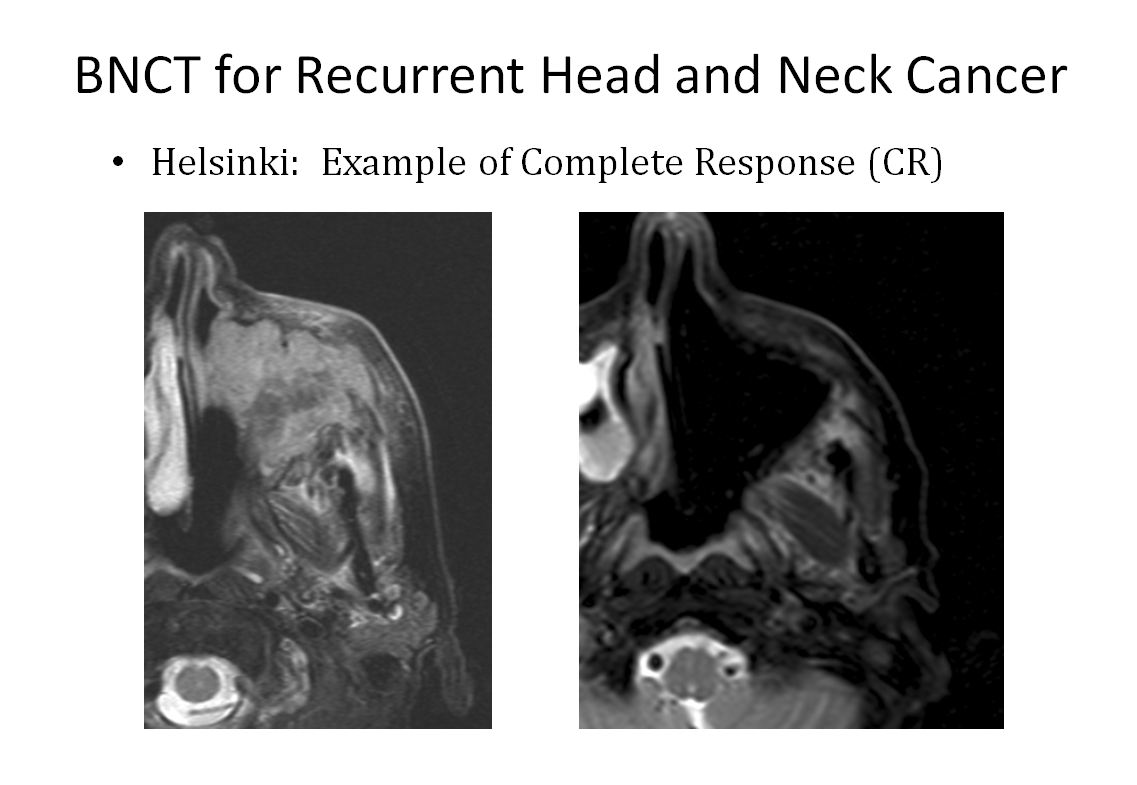
There’s growing clinical work in Japan, a strong revival of interest in the US and the rest of the world, and pre-clinical European initiatives like those at Helsinki hospital. Companies are rapidly developing new devices and boron-10 delivery drugs, and oncologists worldwide are taking notice.
History of Boron Neutron Capture Therapy
Dr Paul Busse, the Joseph W. Cotchett Endowed Chair in Radiation Oncology at Massachusetts General Hospital, explains the basics of radiation therapy (RT) and the historical evolution of BNCT efforts.
Conventionally, oncologists treat patients with photon or proton radiation. With X-ray RT sources, there’s a limit on the depth and complexity of a tumour that can be successfully treated. On the other hand, proton therapy (PT) is a more precise way of delivering radiation than conventional radiation therapy.
A PT beam has a very sharp beam edge and can penetrate tissue to a depth determined by its energy, at which it deposits most of its energy as defined by its characteristic Bragg peak.
There’s only one way to deliver radiation with a dose distribution conforming closely to the shape and volume of the tumour – and that is to irradiate cancer cells from the inside out.
That’s the promise of boron neutron capture therapy, a modality of biological targeting and radiation delivery. In BNCT, a low energy neutron is selectively taken up by boron-10 (10B) atoms, while other atoms of other elements also absorb it, but to a lesser degree.
The nuclear reaction is ![]() .
.
The 10B (boron-10) atoms instantly decay into an alpha particle (4He), a recoiled lithium nucleus (7Li), and a γ particle.
Cancerous Cells Are Killed From the Inside
Dr Busse notes that these particles traverse tiny distances before giving up their tremendous kinetic energy. It is deposited where we want it – ideally within the cell nucleus, within the range of macromolecules.
An important factor in radiation therapy is radiation protection efficiency (RPE) which qualifies the effectiveness of a shielding material or the difference in the radiation uptake between the targeted (cancerous) and healthy cells. RPE is defined for ortho-voltage X-rays as 1, while RPE for most boron-10 reactions with today’s delivery drugs is on average 3.8.
We usually talk of three neutron energy ranges – slow (thermal) neutrons, which actually produce the interaction with boron 10, epithermal neutrons of higher energy and fast neutrons, which are, for the main part, undesirable for BNCT.
A good BNCT source produces epithermal neutrons of as homogenous energy as possible to deliver the radiation to deeper targets within the tissue, where their energy drops to thermal levels. Beam intensity is also essential to reduce treatment time and assure greater throughput of patients.
BNCT Started with Nuclear Reactors
The progress of early clinical trials in 1951 and 1960 at the Brookhaven Medical Research Reactor (BMRR) and 1959 at the Massachusetts Institute of Technology (MIT) was slow. The qualities of the beam were far from optimal, and the side-effects for the patients were severe. A new chapter of clinical research opened after that in Japan, led first by Hatanaka and colleagues for curing glial tumours and Mishima, who focused on melanoma and the use of the BPA delivery agent.
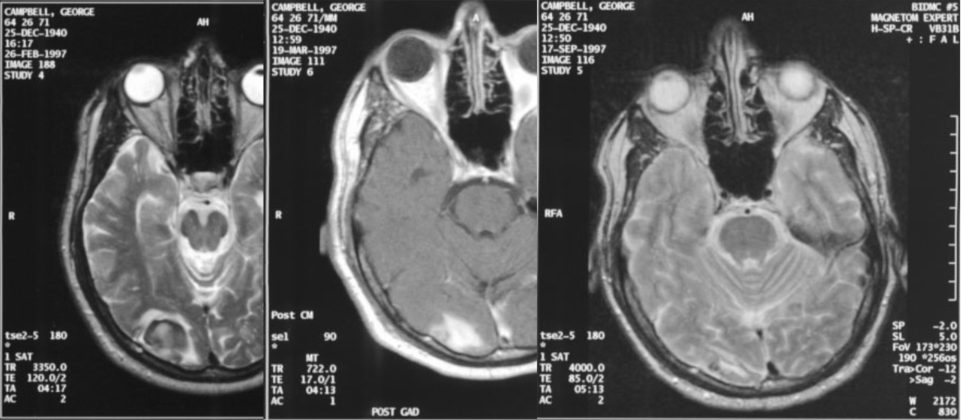
Dr Busse concludes that the most promising results in his BNCT treatment of hidden cancers at the Massachusetts General Hospital (MGH) in the last twenty years have been in treating recurrent head neck cancers. These resist conventional radiation responses, which provide very few good options to patients with the mentioned cancer.
An overview of BNCT clinical studies and practice
As a retired medical professor with thirty years of experience in radiation therapy, Prof. Wolfgang Sauerwein of BNCT Global GmbH notes that all conventional radiotherapy techniques have the same problem: the physician has to define the target volume, and this introduces unavoidable variations in precision and repeatability.
This also implies that unless some entirely new way of delivering radiotherapy is developed, any efficacy improvements in radiotherapy will be incremental rather than substantial.
But boron neutron capture therapy is different. Prof. Sauerwein calls it “a vectorised external beam radiotherapy” that targets single tumour cells and offers the possibility to irradiate the disease, not the tissue volume.
Indeed, nuclear medicine is also looking to irradiate cells with modern alpha emitters, but there is a big difference between BNCT and applying radionuclides.
Alpha-particle therapy targets tumours with selective delivery molecules, but the radioisotopes also reach sensitive organs such as the kidneys.
The Many Indications for Boron Neutron Capture Therapy
Besides head-and-neck cancer, BNCT will play an influential role in many other indications, namely for locally recurrent tumours after a complete regimen of conventional radiation therapy, such as breast cancer local recurrences on the chest wall and after two times of radiotherapy mastectomy.
We also have metastases from uveal melanoma, skin melanomas that are not responding to checkpoint inhibitors, cystic carcinomas, soft-tissue sarcoma, high-grade glioma and malignant meningioma. In essence, these are radio and chemotherapy-resistant tumours with bad patient outcomes.
Prof. Sauerwein says that if we add up all the patients with the abovementioned indications and take into account just the northern hemisphere, with Europe, including Russia, China, Japan, and North America, we have 1.3 million patients per year that can benefit greatly from BNCT, which is a huge market.
But until recently, BNCT was limited to research nuclear reactors and – in Wolfgang’s case – the number of patients he could treat in a decade was 32. Oncologists cannot conduct robust clinical trials with such low numbers of cases.
Thankfully, the situation is changing. In Japan, there are already three hospitals treating patients with accelerator-based BNCT devices inside the clinic, with many more under construction. If we look outside of Japan, Helsinki Hospital is leading the way in Europe in compact BNCT device installation, and accelerator-based BNCT plans are afoot in Russia, China (three centres), Birmingham in the UK, and Mayo Clinic in the US.
Imaging and patient positioning requirements for BNCT
Dr Thomas Rock Mackie, Emeritus Professor at the University of Wisconsin, stresses that for successful irradiation with boron neutron capture therapy, we have to ensure that the malignant tissue is well-boronated. We must achieve sufficiently high tumour-to-blood and tumour-to-normal-tissue ratios of boron-10 concentrations so that the thermal neutrons can deliver a high enough dose to the cancer cells and a meagre dose to normal tissues.
A challenge is that all tumour cells must be labelled, which can be difficult if the tumour’s vasculature is compromised. That is why it may be challenging to treat huge tumours with today’s BNCT delivery drugs.
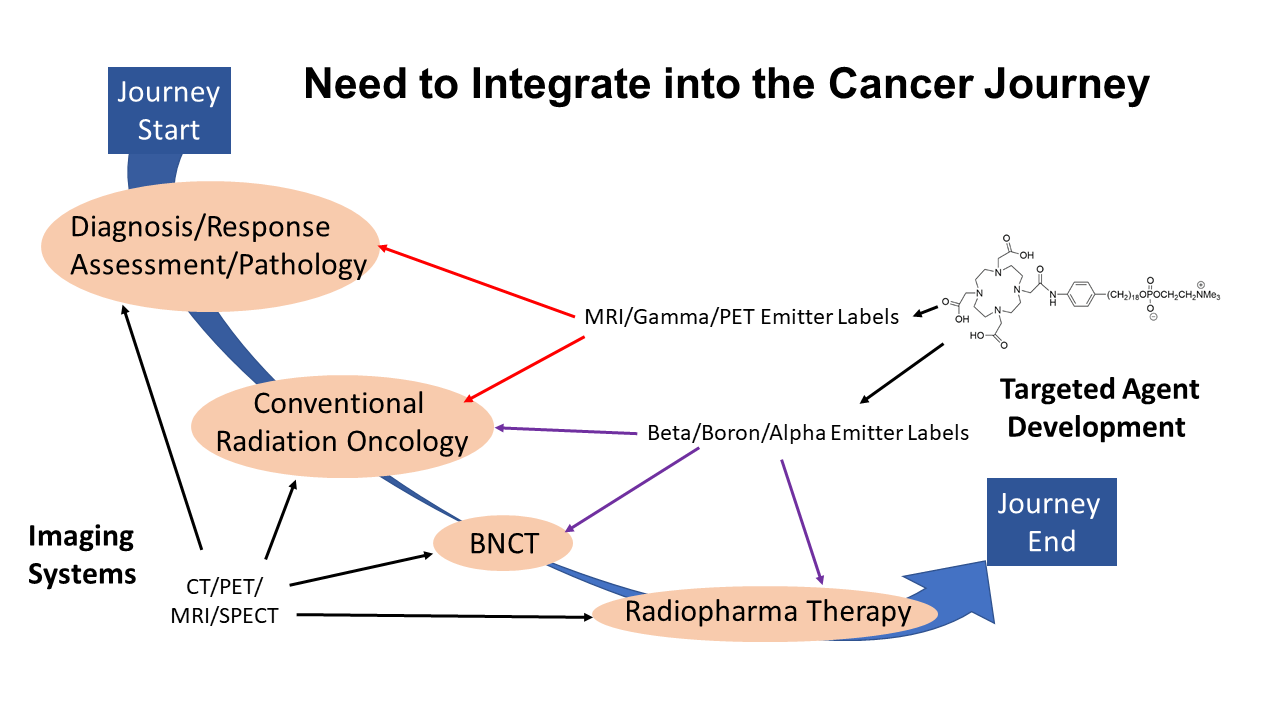
With the new accelerator-based sources of epithermal neutrons for BNCT, treatment planning is becoming increasingly important. The new BNCT devices are expensive pieces of clinical hardware, and it is crucial we gain as much value from them as we can – for example, by being able to treat from multiple directions, choosing targets carefully and avoiding healthy tissue.
Boron Neutron Capture Therapy Must be a Workhorse
Dr Mackie says BNCT should be a workhorse – not only confined to treating the head, neck and brain but, in the future, to be applied to many other parts of the body. We ought to have in-room image guidance to target the beam precisely with the accurate neutron flux and take into account that some tumour parts may be better labelled than the others – for which we have to provide the setup adjustment.
The orientation of the beam-line plays a significant part in treatment planning and patient positioning. Most of the accelerator-based BNCT systems under development will be using a fixed horizontal beam so that they can be placed in a conventional hospital radiation-therapy area, such as the two BNCT system-types from the US.
Dr Mackie believes that BNCT should generally have more flexible patient positioning. Leo Cancer Care, co-founded by Dr Mackie, has been developing a “workhorse” patient-positioner approach to treat any body-site in either the seated (upright) or perched patient position. The company also mounts a dual-energy CT scanner in the therapy room as part of its system, which lets the technicians verify the correctness of the patient setup.
Treatment Planning for BNCT
Dr Hanna Koivunoro is the Chief Medical Physicist at Neutron Therapeutics Inc. During her career she has been involved in BNCT treatment planning and the Finnish clinical trials that treated 250 patients in 300 BNCT fractions.
She notes that BNCT treatment planning has some unique features as it also requires the modelling of nuclear interactions of neutrons. Typically, the clinician has to apply the Monte Carlo simulation of neutron and photon transport within the patient and establish a patient model with elemental composition and boron-10 concentrations of the tissues.
Neutron dose calculation is a bit complicated because all four dose components of boron neutron capture therapy need to be taken into account – boron, nitrogen, photon and fast neutron dose. In addition, there are organ and dose-component specific RBE (relative biological effectiveness) and CBE (compound biological effectiveness) factors to consider.
Biologically Weighted Doses Matter in BNCT
Considering typical BPA drug-uptake in the brain, the boron dose is the highest component. The second is the photon dose, and then nitrogen and fast neutron dose components come at the low end. However, these physical dose components do not denote much about the biological effect of BNCT until they are multiplied by RBE/CBE to produce a biologically weighted dose because the latter is dependent on the boron track applied.
The neutron source and the beam shaping assembly around the neutron source are typically modelled with a Monte Carlo simulation. Then the neutron energy and angular distribution are recorded inside the collimator or at the beam port, and the energy spectrum is verified by measurements.
The accelerated protons of the BNCT device hit the Beryllium target that produces the neutrons then there is a beam-shaping assembly that slows down the neutrons to epithermal energies.
Patient modelling in BNCT
For BNCT, patient modelling considers the most important organs that need to be contoured, such as skin, bones, brain, lung, air cavities and brain, including the spinal cord.
Oncologists typically use MRI (magnetic resonance imaging) and PET for determining the macroscopic tumour GTV (gross tumour volume), with the addition of a margin for sub-clinical disease, spread to define the CTV (clinical target volume), so that CTV = GTV + margin. Dr Koivunoro points out that, at least in Finland, clinicians use a margin of at least 1.5 centimetres for BNCT, applying a homogeneous composition to each region.
Generally, oncologists have to model boron-10 concentrations correctly within 20% to 30% for accurate neutron transport.
Only skin and mucous membranes have a more significant boron-10 uptake than cancer cells, so the treatment plan must direct patient positioning in such a manner that these tissues are irradiated as little as possible.
Good responses to BNCT treatment after one fraction
Because we can modulate the neutron field in accelerator-based BNCT devices, we can often achieve a relatively homogenous dose in large tumours. On the other hand, we currently do not yet know the optimal BNCT dose for killing cancerous cells. Hence, clinicians prescribe relatively high doses based on the tolerance dose of normal tissues.
Nevertheless, Dr Koivunoro emphasises that – in contrast with traditional radiotherapy – patients with cancer types indicated for BNCT have typically excellent responses to BNCT treatment even after one fraction, dose-volume histograms showing parts of the tumour receiving, for example, doses of 10 Gy to 35 Gy, while the spinal cord irradiated with less than 4 Gy.
Status of Boron Neutron Capture Therapy in Japan
Dr Akira Matsumura is the president of Ibaraki Prefectural University of Health Sciences and a neurosurgeon who entered the radiation therapy field early on and has considerable experience with both proton therapy and boron neutron capture therapy.
He recalls how, for years, Japanese oncologists used two reactors in Japan, the Tokai (JAEA JRR-4) and Kyoto (KURRI, KUR), of which the former was shut down due to the earthquake, and the latter will be shortly closed.
Japan has several accelerator-based BNCT facilities currently up and running, the most of any country in the world. Two clinics and one university have a cyclotron-based BNCT device, specifically the Osaka Med & Pharm University, Southern Tohoku BNCT research center and Kyoto University.
The second type of a clinically operating BNCT device is the one in the National Cancer Center Hospital in Tokyo, which has a high current of low-energy protons. This device has, interestingly, a vertical beam. It uses a LINAC to generate its protons which slam into a lithium target to produce epithermal neutrons.
The third class of BNCT system is installed at Dr Matsumura’s “home base” at the University of Tsukuba. It is also based on a LINAC, with a medium proton energy of eight MeV and a medium proton current of 2 mA. The device employs a barium target and a horizontal beam.
New Japanese BNCT Facilities Under Construction
As proof of what a BNCT hotbed Japan really is, three additional facilities are currently under construction – or nearing it. These are the Edogawa Hospital BNCT Center, the Shonan-Kamakura Hospital and the Nagoya University.
The Shonan-Kamakura device uses an electrostatic accelerator to produce a low-proton beam with a very high proton current and has a lithium target. The Nagoya University BNCT facility will also use an electrostatic-based device with a lithium target, but with half of the Shonan-Kamakura’s proton current, namely 15 mA.
Summary of ongoing Japanese clinical trials
With all its investment in BNCT technology, it is little wonder that Japanese oncologists probably lead in the number and scope of BNCT clinical trials.
The Recurrent Malignant Glioma (JG002) trial in Osaka was closed in 2020 and is now moving into phase II (single arm) – comparison with historical data. Its phase I was very successful, as the team reached the patient median survival time of 18.9 months in the studied indication.
The Malignant Melanoma & Angiosarcoma trial (JapicCTI-195062, Phase 1) started in 2019 and is ongoing, while the Malignant Meningioma trial (jRCT2051190044), also commenced in 2019, is in full swing. The latter is a randomised controlled trial of 18 cases, also headed by the Osaka medical and pharmaceutical University.
It is notable that the Japanese national health system approved BNCT treatment for recurrent head-and-neck cancer in 20202 and which is now subsidised by at least 70 %, the total cost being about 4.1 million Japanese yen or 32,673 EUR.
Dr Matsumura points out the same as many other BNCT experts – for the future expansion of clinical indications for BNCT, global clinical trials with cooperation and information sharing between BNCT facilities all over the world are essential.
The future of BNCT in every clinic
It is fitting to end the clinical-aspects summary of the webinar by highlighting the words of Prof. Wolfgang Sauerwein, who was the moderator of this part of the webinar. He notes that to fully succeed as a routine ray-burst modality for treating cancer at your local oncology clinic, modern BNCT must become reimbursable by the national health systems and insurance companies worldwide.
The only way to achieve this is by proving, through combined clinical trials with merged BNCT evidence, that BNCT “performs as designed”. This hybrid radiotherapy modality will succeed clinically when the BNCT community achieves critical mass by working economically and collaboratively in treatment protocols, staff training and data exchange.
More, perhaps, than in any other field of modern radiation therapy, the rallying cry “Together we stand” holds true for the community of boron neutron capture therapy.
The webinar “Today’s Use and Future Aspects of Boron Neutron Capture Therapy” is rich in detail and subject matter. This blog offers just a summary of some of the talking points regarding clinical aspects. See also the blog that covers Part II of the webinar, in which we examine the participating experts’ views on industrial development and challenges of BNCT.
List of the experts participating in the educational webinar on BNCT on the 2nd of March 2022 (in order of appearance)
- Jay Flanz, PhD (President and Chair of PTCOG, Retired assoc. Prof. at the Harvard Medical School, Retired Technical Director at the Francis H. Burr Proton Therapy Center of the Massachusetts General Hospital);
- Paul Busse, MD, PhD (Joseph W. Cotchett Endowed Chair in Radiation Oncology, Massachusetts General Hospital);
- Wolfgang Sauerwein, Prof. Dr Med (BNCT Global GmbH, Heinrich Heine University Dusseldorf, President of the German Society for Boron Neutron Capture Therapy);
- Thomas Rock Mackie, PhD (Emeritus Professor, University of Wisconsin, Board Chairman and Co-Founder of Leo Cancer Care, Board member of Cosylab, Board member in Shine Medical Technologies);
- Hanna Koivunoro, PhD (Chief Medical Physicist at Neutron Therapeutics Inc.);
- Akira Matsumura, M.D. Ph.D. Dr.med (President, Ibaraki Prefectural University of Health Sciences, Emeritus Professor, Faculty of Medicine, University of Tsukuba, Japan);
- Toshinori Mitsumoto, Principal Engineer at Sumitomo Heavy Industries;
- Elizabeth Reczek, PhD (Chief Executive Officer at Neutron Therapeutics, Inc.);
- Rob Hill (Chief Operating Officer of TAE Life Sciences);
- Seppo Pakala (Chief Medical Officer at Neutron Therapeutics Inc.);
- Niek Schreuder, PhD (Chief Scientific Officer at Leo Cancer Care);

