Designing, Operating and Financing a BNCT Center

In November 2022, Cosylab and Deerfield Management organised a comprehensive webinar with a panel of four experts from Japan, Italy, Finland and the US. They shared their experience regarding planning, financing, construction and operation of a BNCT centre.
The panellists were, in order of appearance, Naonori Hu from the Kansai BNCT Medical Center in Osaka, Sandro Rossi from the National Centre for Oncological Hadrontherapy (CNAO) in Pavia, Mikko Tenhunen from the Helsinki University Hospital, and Avi Kometz from Deerfield Management.
Where is BNCT Already Thriving?
BNCT is the hybrid therapy modality that combines neutron radiation sources and boron-10 delivery drugs, such as BPA (boronophenylalanine). Combining high doses with cell-selective radiation, BNCT is the therapy with the lowest impact on the patient’s quality of life compared to other radiation, chemotherapy or biological therapy modalities, as it demands at most two fractions.
Today, Japan is one of the clinical BNCT powerhouses, with years of accomplished clinical trials and successfully operating several fully-functioning clinics, such as at Osaka. In 2020, the Japanese national health insurance system approved reimbursement treatments for recurrent head and neck cancer treatment.
Approximately 2000 patients have been treated worldwide with BNCT, and there are about sixteen BNCT centres based on compact accelerator designs in various stages of development around the world.
Europe is also building its new BNCT centres, the first being in Finland. The BNCT centre at the Helsinki University Hospital, with a room-sized proton accelerator, is already operating daily for verification and validation testing. The first clinical trials are expected to begin in late 2023. Other BNCT facilities are planned or are being built in Italy (CNAO), the United Kingdom, Spain, Poland and Germany.

What Staff do we need for a BNCT Clinic?
A BNCT clinic needs a staff of various professions. Radiation oncologists, nuclear medicine radiologists (involved with the radiation dose prescription and consultation) and radiologists comprise the doctoral lineup, each of them tending to his specific field. The radiologists, for example, perform medical imaging of boron uptake through the F BPA or F FDG (fluorodeoxyglucose F 18) drugs which play a central role in today’s BNCT.
The clinic also needs medical physicists and dosimetrists to plan the treatment and support ongoing QA & QC, and perform research and development. Radiation technologists and radiation therapists perform the patient’s setup process using fixation-immobilisation devices and assist with QA & QC.
The centre should also have a pharmacist who operates the FDG PET drug and measures the boron-10 carrier concentration in the patient’s blood which is required for the CT dose calculation. Lastly, the nurses, reception staff and the cyclotron operator round up the genuinely multidisciplinary team that cares for the BNCT patients.
Two Examples of Finished BNCT Facilities
Naonori Hu described his clinic’s Sumitomo Heavy Industries BNCT device at Osaka, based on a 30-MeV-proton cyclotron and a beryllium target. It took the Kansai centre two years, from building and installing equipment to its opening in 2018. The facility consists of the patient preparation room — where the patient is set up according to the treatment plan –, the treatment room with the automatic patient-transfer system, and the control room.
At Helsinki, the Finnish team has roughly the same segmentation of rooms in their BNCT facility as the Osaka clinic has. The Helsinki device is a Neutron Therapeutics machine (nuBeam) with a 2.6 MeV-proton electrostatic accelerator and a rotating, solid lithium target for generating neutrons. Helsinki University Hospital (HUH) started test-using the nuBeam system in January 2019 and will begin clinical safety trials in 2023.
Mikko Tenhunen from HUH noted they expect to treat the same kind of cancers as the Osaka team and will have all the necessary multi-profile personnel present during an eight-hour working day for BNCT, aiming to process up to four patients per day.
The treatment room at Helsinki is about the same size as its conventional radiation therapy equivalent. The CT scanner in the room is mounted on a rail, to be parked behind a protective door during neutron irradiation. A robotic couch is used for patient positioning. The heavy-concrete bunker is located inside the existing clinical building, where 230 m2 of space was allocated for the device’s equipment and 110 m2 for ancillary use.
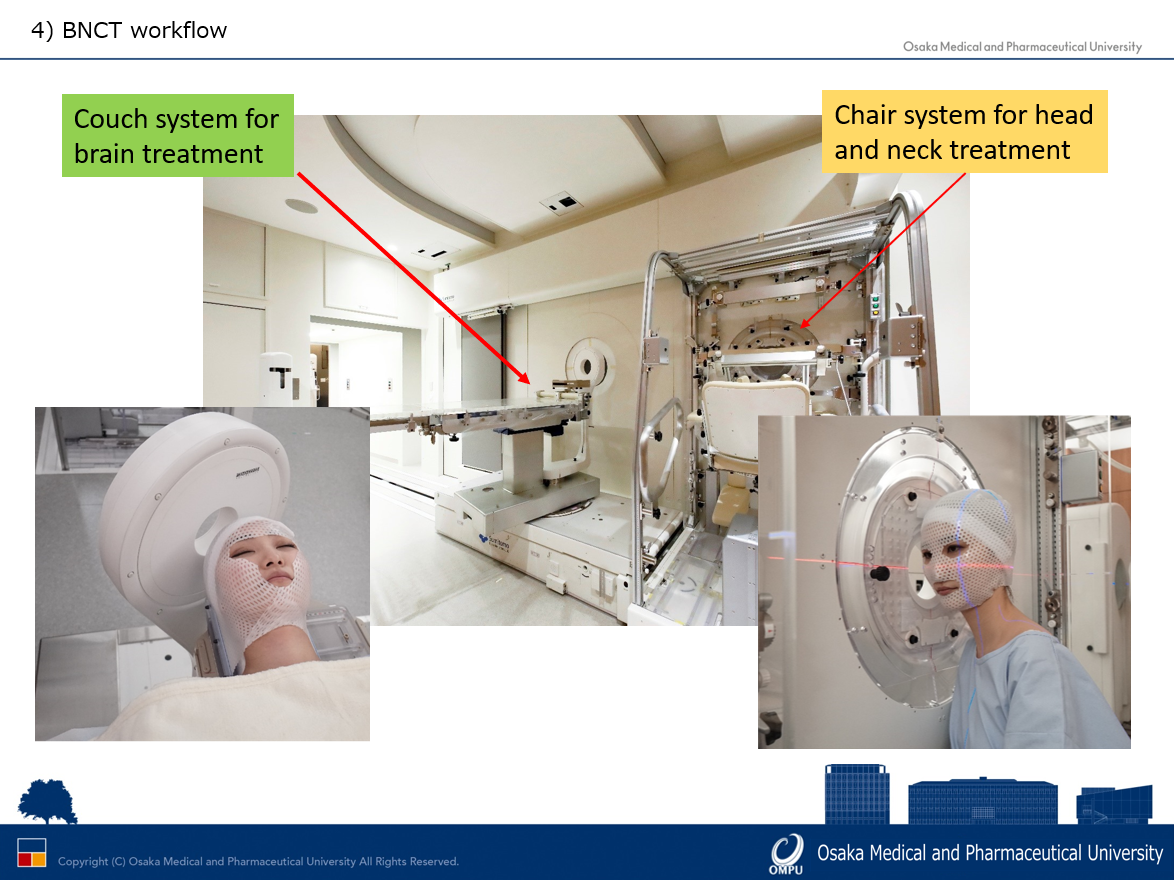
How does BNCT’s Workflow Look?
Naonori Hu explains that the workflow of BNCT at the Kansai BNCT Medical Center starts with pre-planning based on the patient referral from another medical institution, with the whole process taking no longer than two weeks. The Osaka team uses the received CT or MRI to perform pre-calculation to verify whether BNCT is indicated for the particular patient. If it is, and the “head-and-neck” consultation is positive, then they move to the preparation stage, simulating patient positioning and imaging in several steps. One to two weeks before treatment, the team performs a PET CT scan of the patient – called an “F BPA PET”– so they make sure the patient can be treated with BNCT. F BPA PET shows the boron uptake in the patient’s tumour before they had been treated. If the uptake is sufficient, the team moves to the next step, the patient-positioning trial — usually one week before the actual session.
The trial is similar to the X-ray verification for conventional (photon) radiation therapy, where technicians use a thermoplastic mask to immobilise the patient’s positioning. The team also performs a CT scan which is to be used for the final treatment planning process. In BNCT, particularly for head-and-neck treatment, it is often challenging to acquire the correct angles for specific patients. The imaging for this may take even an hour or two, although well-versed teams can shorten this average with time. After all this, the team creates the patient treatment plan.
The Osaka facility uses two couch orientations, depending on the location of the patient’s tumour – either the supine-on-couch for brain cancer or the couch-chair for head-and-neck cancer.
On the specific day of the treatment, a technician initiates the boron-10 infusion for the patient two hours before irradiation. After an hour has passed, the team positions the patient, and a radiology technician performs X-ray imaging to verify the setup. Immediately before targeting with neutrons, the team takes a final blood sample to confirm the blood-boron concentration that determines the radiation time. After the BNCT treatment, the patient returns to the hospital generally after 24 to 48 hours for a check-up.
An Example of a BNCT Facility in Construction – CNAO
Sandro Rossi from the National Centre for Oncological Hadrontherapy (CNAO) explained his organisation was adding several preparation areas and two BNCT treatment rooms to its existing hadron therapy facility. The addition takes up roughly 25 by 25 square metres of floor space, with the device’s accelerator being placed between the two treatment rooms.
To start with, the Italian team will be using one room for human patients. The other room is slated mainly for experiments with small animals, so the CNAO facility has two transport lines. These lead from the TAE Life Science tandem accelerator, emitting protons at 2.5 MeV and with a current of 10 to 15 mA, towards the two neutron-emitting lithium targets.
The beam should become available in late 2024. As CNAO also operates as a clinic within the Italian National Health System, BNCT therapy will also be free of charge for its patients.
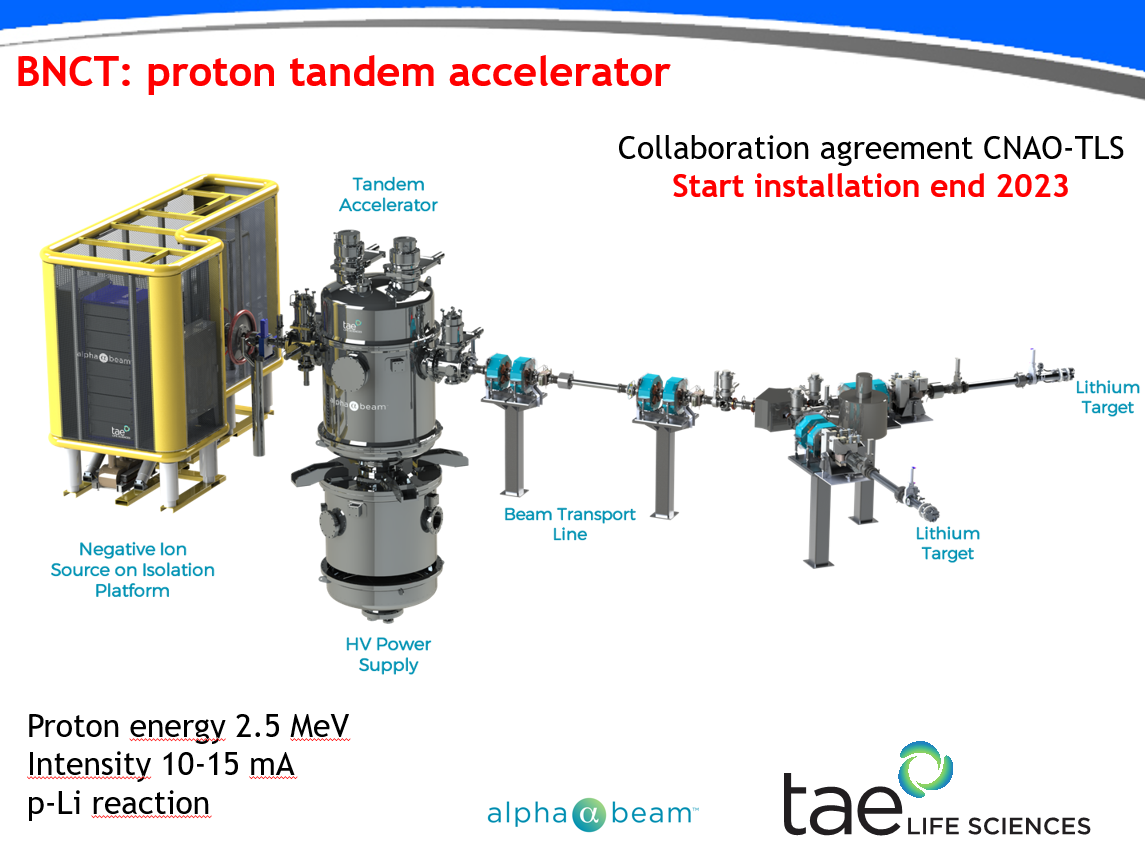
Note: “TAE Life Sciences’ Alphabeam™ is in development and not available for sale or commercial use”.
Why Invest in BNCT Centres?
Why should investors be interested in boron neutron capture therapy? Firstly, BNCT can cure or alleviate certain types of cancers that other therapy modalities have not yet been able to date. This will solidify BNCT as a disruptive radiation therapy, not just an incremental one.
Secondly, the spectrum of tumours BNCT could treat is broad, increasing its cancer-treating utility. Thirdly, BNCT can be used outside oncology in other therapeutic areas. As BNCT is a complex system of devices, software, drugs, and healthcare provision, there are potentially many ways for clinics and investors to reap their respective rewards.
When to Invest in BNCT?
Avi Kometz from Deerfield Management underlined that embracing novel radiation therapy technology can present both a risk and an opportunity. For some medical centres, it may be beneficial to be an early adopter of BNCT, as they would be able to treat very challenging tumours sooner. It also enhances a centre’s competitive advantage, becoming a referral institution for treating difficult cancers. At the same time, it enables the radiation oncology team to pursue leading-edge research programs, such as alpha-particle therapy.
Operating BNCT in a medical centre can be profitable overall, although planners must vet the technology beforehand to confirm its risk-benefit profile. Therefore, early adopters should weigh and analyse the risks inherent to their case, preparing mitigation scenarios, such as repurposing the BNCT centre as an alternative — for example, as a proton therapy facility.
What is the Construction Timeline of a BNCT Centre?
When a hospital starts establishing a BNCT centre, it is probably four years away from fully treating patients in a clinical program. The first year is usually dedicated to planning, while the next 12 to 18 months are devoted to site construction or adaptation. Around two years is needed for system installation, a part of which can begin before the work on the site is complete, running in parallel. Lastly, the centre commissioning can take anywhere between six and eighteen months, depending on local regulations and other legal considerations.
How Much Does a BNCT Centre Cost?
Broadly, the costs of a BNCT centre consist of two parts. The first covers the initial setup costs, while the second defines the annual operating expenses. The financing of the upfront – or setup — costs of a BNCT centre in the US comes to 40 to 50 million USD. The latter covers the procurement and commissioning of the accelerator-based BNCT device and other equipment, the facility construction and the equipping of the centre.
A representative estimate for the BNCT device is about 25 million USD, which includes the proton accelerator, the software, the patient bed and robotics, the imaging equipment and the treatment room itself. The construction costs depend on whether the facility is a greenfield or is a repurposed existing facility, so they run between 15 to 25 million USD.
The annual costs, on the other hand, consist of running and maintaining the “BNCT accelerator”, the staffing expenses, and all of the daily-operating costs. Avi Kometz estimated the annual operating expenses of a BNCT centre in the United States to be from 9 to 12 million USD, of which the part covered by the service and warranty agreement between the clinic and the accelerator vendor would be between 2 and 3 million USD.
The staffing costs for the medical centre would include expenses for the professional workforce providing the healthcare, administration and management services. These would run at about 6 to 8 million USD a year. The costs for the daily operations and utilities, such as marketing, would be in the vicinity of a million USD annually.
Reimbursement of BNCT
The total BNCT radiation fee in Japan comes to approximately 20,000 USD. The National health insurance covers the additional price of the boron-10 drug, so the patient pays for one fraction, out of their pocket, the equivalent of 1,000 USD.
Most experts agree that patient volumes will not be a limiting factor in the early decades of BNCT for the growth of the technology and number of centres.
The number of patients annually to be treated before FDA approval in a US centre is estimated at 140 patients in clinical research programs, providing the clinic with annual revenue between 3 and 4 million USD. The patient numbers would increase after the FDA approval, up to 1,000 unique patients annually. The latter phase could bring in an income of 25 million USD a year, improving profitability.
Towards a Future with BNCT in your Local Clinic
An increasingly ageing population, and large backlogs from the COVID-19 period, are putting pressure on the healthcare sector worldwide to increase the effectiveness of anti-cancer treatments and lower costs and therapy time.
There is a substantial global market opportunity for new cancer treatment modalities. The statistics of half a million new patients annually in Europe, China, Japan, and the US show a market potential for at least 1,800 BNCT devices to be clinically operating globally in twenty years. This is based on the current applicability of BNCT to twenty per cent of cancers – which will undoubtedly rise in the future.
The global opportunities are enormous — for manufacturers of BNCT devices, software and delivery compounds, healthcare investors and oncology departments of hospitals and campuses everywhere. But in the end, what matters most are the patients. BNCT can ensure that cancer patients with some conventionally untreatable tumours can have a relatively short therapy with a higher probability of recovery and of returning to their everyday life.
List of the experts participating in the webinar on 9th of November 2022 (in order of appearance):
- Niek Schreuder, PhD (Chief Scientific Officer at LEO Cancer Care); moderator of the panel and webinar;
- Naonori Hu, PhD, Assistant Professor at Osaka Medical and Pharmaceutical University Kansai BNCT Medical Center;
- Sandro Rossi, General Director of The National Centre for Oncological Hadrontherapy (CNAO);
- Mikko Tenhunen, Professor, Chief Medical Physicist, and Head of Division at Helsinki University Hospital;
- Avi Kometz, MD, Medical Technologies Partner at Deerfield Management.
