
Interview: How will a New Gastric Monitoring System Improve the Outcomes of Critically ill Patients

We asked Mr. Nico Van Tichelen, CEO at VIPUN Medical to describe the company’s mission, to talk about the benefits the VIPUN Gastric Monitoring System brings to doctors and patients, and how he sees the development of the medical device industry as a whole.
Mr. Van Tichelen, can you tell us a bit about VIPUN Medical?
VIPUN Medical is a medical technology start-up located in Mechelen, Belgium. Our objective is to support health care providers globally in improving the health of patients through the optimal administration of nutrition, while striving to relieve doctors and nurses from some medical nutrition safety concerns which they currently face.
What is the story behind the development of a novel Gastric Monitoring System?
The technology behind the VIPUN Gastric Monitoring System was developed by Dr. Pieter Janssen at the University Hospital in Leuven, Belgium.
Dr. Janssen has been involved in gastro-intestinal research in animals and humans for more than 20 years, and he recognised that there is no available easy-to-use medical device which provides accurate and continuous insight into gastro-intestinal function, or more specifically the stomach’s motility.
Impaired gastric motility is, however, an important factor in different gastric diseases and food intolerance in general.
How will the new device influence doctors’ work? How will it help patients?
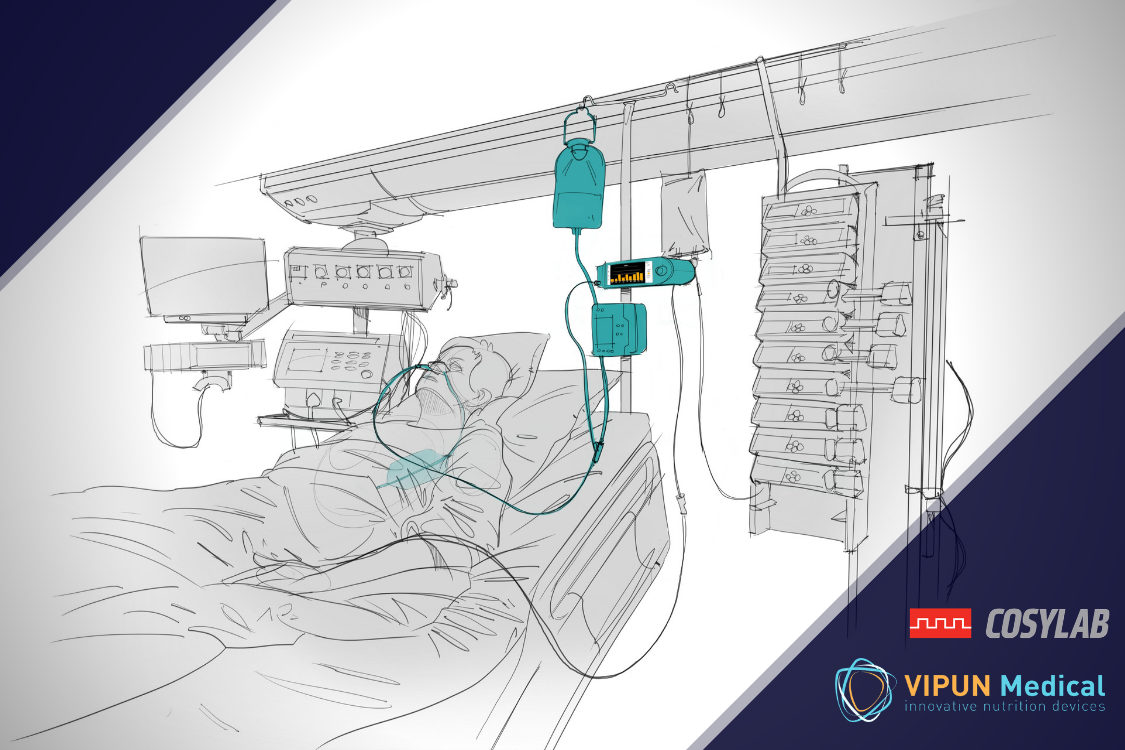
Monitoring stomach motility is especially important when patients must be fed through a nasogastric tube (enteral nutrition), as is often done in at the intensive care unit or after abdominal surgery.
While medical associations strongly recommend feeding critically ill patients enterally when no obvious contraindications are present, physicians and nurses are often reluctant to do this as they are afraid patients will develop feeding intolerances that cause all kinds of complications.
As a result, patients in the ICU are often malnourished with worse clinical outcomes as a final result.
What do you see are the benefits of partnering with Cosylab?
Cosylab was able to convince us very quickly by bringing us in touch with the team that will actually do the job. We are impressed by the high quality people on the team and the feel each team member has for accountability.
While we did of course only contact potential partners with all the right certifications in place, we feel that Cosylab is the most up to speed on specific medical device quality standards, and we are confident the execution of this project by Cosylab will lead to the regulatory approval of our device.
Is there anything you would like to share with innovators and startups about their development journey?
Like in any project, but specifically for medtech start-ups, it is crucial to engage with the right partners. The life science environment is complex and regulatory requirements are becoming increasingly tough. The primary job of the start-up management team is to seek those partners who can support you in delivering an innovative technology that answers the medical need, as well as the regulatory requirements. As we can not focus on all aspects of such a large scale project at once, you have to find the right partners and trust their capabilities to do their part of the job.
We invite you to read the official Press Release Cosylab and VIPUN Medical to Improve the Outcomes of Critically ill Patients.
